
My research activities focus on the development of novel dense collagen-based biomaterials for tissue engineering and the controlled release of biomolecules. The targeted applications are numerous. First, we are developing composite hydrogels combining type I collagen with biodegradable synthetic polymers or nanoparticles for the development of novel medicated dressings to treat cutaneous chronic wounds. The aim is to release active molecules, cytokines or therapeutic genes in a controlled manner in order to promote wound healing. My second activity is devoted to the development of in vitro 3D models for the study of pathologies and the testing of new pharmacological molecules. We are focusing on the development of cardiac models or biomaterials mimicking the structure of the intervertebral disc by following a biomimetic approach. We aim to synthesize a matrix acting as a support for cells which would possess the physical and biochemical properties of the natural extracellular matrices of these two organs. The ultimate goal is to recreate the 3D environment of cells and study their behavior in a physiological context.
Project 1
Novel three-dimensional models for the study of dilated cardiomyopathies: dense anisotropic and macroporous collagen hydrogels
Dilated cardiomyopathies are pathologies affecting the heart and lead to heart failure. When Pharmocological treatments are not effective any more, the only treatment is heart transplantation. These diseases are in 40% of cases of genetic origin and are often orphan diseases because more than 100 genetic mutations can be at the origin of this pathology. With the aim of better understanding this disease and to test new pharmacological molecules, an in vitro 3D cardiac model is of great interest.
Our strategy is to synthesize a biomaterial with the physical properties of the extracellular matrix of the heart in order to cultivate the cells of patients affected by dilated cardiomyopathies. Our biomimetic approach is to recreate the three-dimensional environment of the physiological cardiac extracellular matrix to analyze the behavior of diseased cardiac cells. We are developing a dense macroporous and anisotropic collagen hydrogel with adequate mechanical properties using dense collagen 3D printing (Figure 1A). Our physicochemical study determined the conditions for collagen gelation to maintain the alignment of collagen fibrils (anisotropy) while preserving important mechanical properties. In addition, we have developed a process to generate macroporosity within the hydrogel during 3D printing (Figure 1A) or by adding needles. The biomaterial thus obtained has a macroporosity which makes it possible to cultivate cardiomyocytes, the cardiac cells, and to introduce a perfusion system to maintain cell survival (Figure 1B). Initial cell colonization tests showed that macropores could be colonized by cells to recreate the 3D organization (Figure 1C).
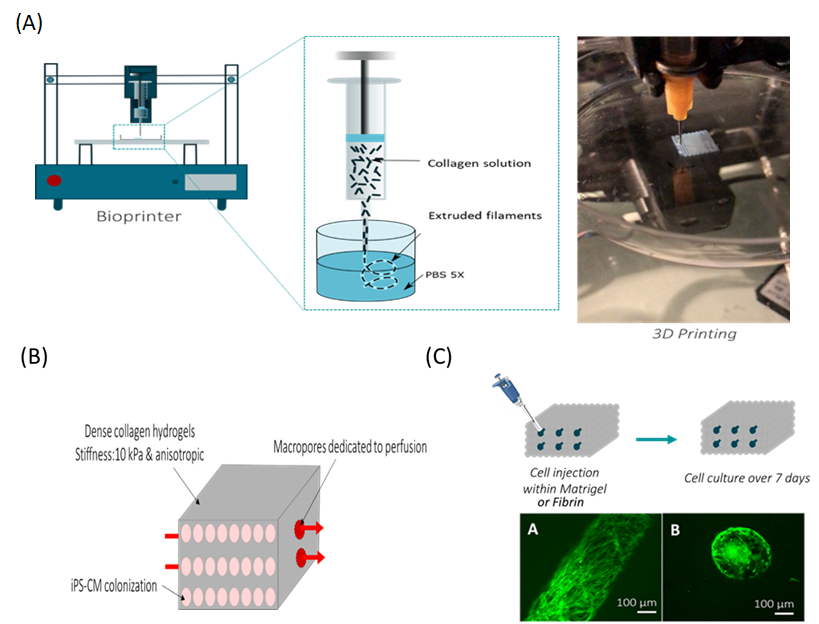
Figure 1: Fabrication of dense anisotropic collagen hydrogels as an in vitro 3D cardiac model for the study of dilated cardiomyopathies. (A) The hydrogel supporting the cardiac cells is produced by 3D printing. (B) The hydrogel thus obtained must be anisotropic, have a stiffness of 10 kPa and be macroporous to allow cell culture. (C) Injection of fibroblasts into macropores for hydrogel colonization.
Project 2
Dense collagen / polyester composite hydrogels for controlled drug release: a novel medicated dressing for the treatment of cutaneous chronic wounds.
Chronic skin wounds are a consequence of unbalanced diabetes, which occurs in 15% of patients. Usual treatments based on ccompression methods or the utilization of alginate hydrogels may not be effective enough to heal these wounds. There is therefore great interest in a new medicated dressing that would speed up skin wound healing. Collagen hydrogels are promising candidates because they have healing properties.
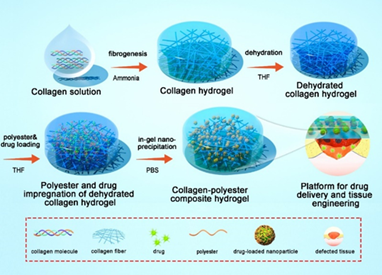
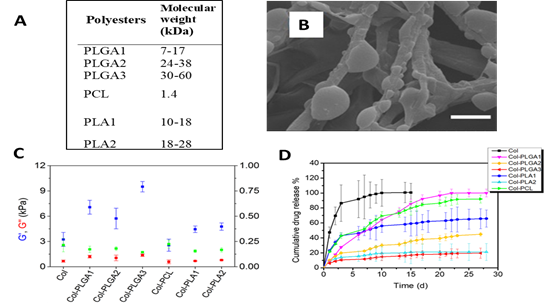
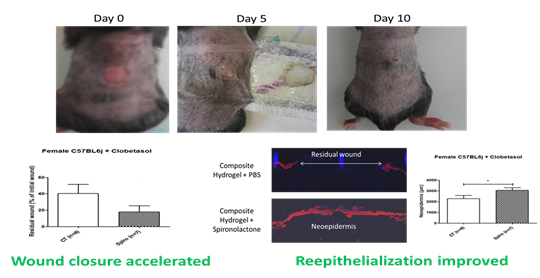
We have developed an original method to synthesize dense composite hydrogels combining type 1 collagen and a drug delivery system. Our strategy is based on the in situ precipitation of polyesters and pharmacological molecules within the collagen 1 network (Figure 2). A dense collagen hydrogel is formed by raising the pH. After a step of dehydration with tetrahydrofuran, hydrogels were incubated overnight with a mixture of polyesters and pharmacological molecules. The following day, the precipitation of polyesters encapsulating the drugs was carried out by immersion in a non solvent. This technique is efficient to immobilize a large quantity of polyesters and drugs in the hydrogel and allow the controlled release of the active molecules. Several types of polyesters were tested (Figure 3A). Composite hydrogels prepared with 7 kDa PLGA (PLGA 1) possess improved mechanical properties (Figure 3C) and a fibrillar structure in which PLGA particles have precipitated (Figure 3B). The release of spironolactone, drug favoring wound healing, is sustained and constant over about a month (pink curve – Figure 3D). An in vivo test in mice was performed. Composite hydrogels have a positive effect on cutaneous wound healing by accelerating wound closure and promoting the formation of new epidermis (Figure 4).
This research program was funded by the Agnece Nationale de la Recherche: Collag-Heal project (ANR-14-CE16-0010) and resulted in a patent filing (US20190151495A1)
Project 3
Injectable collagen / hyaluronic acid composite hydrogels for the regeneration of the intervertebral disc
Back pain is considered as “the evil of the century “with a prevalence that increases with the aging of the population. In half of the cases, the low back pain is due to the degeneration of the intervertebral discs (IVD). These are located between each vertebra in the column and play an important role in the mobility of the trunk. Nucleus Pulposus (NP), located at the IVD centre is gelatinous and acts as a hydraulic damper. It is surrounded by Anulus Fibrosus (AF), fibrous tissue that helps to contain the pressure exerted on the Nucleus Pulposus (Figure 5). In the case of degeneration, the NP becomes dehydrated and can leak by cracking the AF creating a herniated disc (Figure 5).
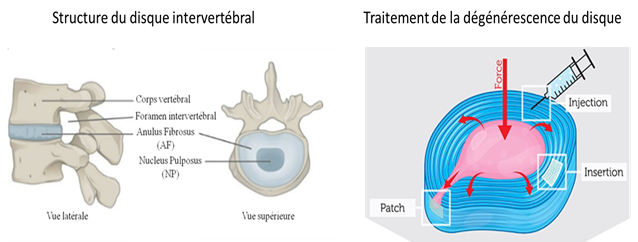
Our biomimetic strategy aims to develop a composite hydrogel composed of type 1 collagen and hyaluronic acid in order to reproduce the biochemical, physical and biomechanical properties of Nucleus Pulposus. By injecting this hydrogel, it would be possible to restore the physical properties of NP and to encapsulate stem cells in it, which could promote the regeneration of the intervertebral disc. On the other hand, this biomaterial could be used as a 3D in vitro model of NP to understand the early stages of degeneration. With the help of a physicochemical study, we analyzed the interactions between the two biopolymers and developed a suitable formulation to mimic NP (Figure 6). Using a collagen / hyaluronic acid, used with a ratio of 1: 5, we reproduced the mechanical properties and hydration of NP. Moreover, its biochemical composition and its ultrastructure resemble those observed in vivo. Finally, it is possible to encapsulate mesenchymal stem cells in biomaterials without affecting their viability.
This research program is funded by the ANR for the development of an intervertebral disc model for the study of the IVD degeneration and the test of pharmacological molecules. (INDEED project, ANR-19-CE06-0028)
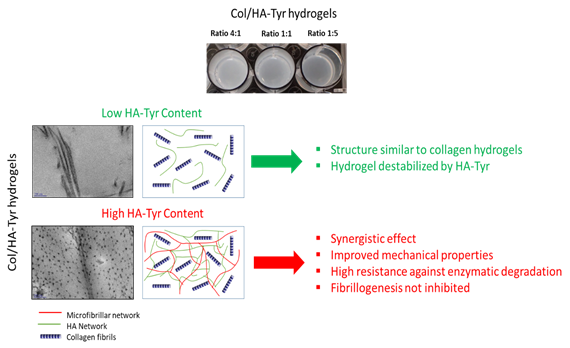
Figure 6: Composite Collagen / Hyaluronic Acid hydrogels for the treatment of Nucleus Pulposus. A high content of Hyaluronic Acid (HA-Tyr) improves the mechanical and hydration properties of hydrogels.
