
Laboratoire de chimie de la matière condensée de Paris
Tour 44-43 / 4ème étage
Case courrier 174
4, Place Jussieu
75005 PARIS
France
Dr. François RIBOT
Directeur(trice) de Recherche
NANO
francois.ribot(@-Code a retirer pour éviter le SPAM-)sorbonne-universite.fr
bureau 34-44-418
François Ribot was born in 1962. He graduated from Ecole Supérieure de Physique et Chimie Industrielle (ESPCI) in 1986. He then received his PhD in inorganic chemistry in 1990 from Pierre et Marie Curie University (UPMC), now part of Sorbonne University. His work, performed under the supervision of Pr. C. Sanchez and financed by Rhone-Poulenc Company, dealt with the sol-gel process of yttrium(III) and cerium(IV) oxides. During his PhD, he spent 16 months as a research associate in the group of Pr. Egon Matijevic at Clarkson University (Potsdam, NY, USA), working on the synthesis of uniform sub-micronic mixed colloids of yttrium(III) and copper(II) compounds. He joined the CNRS in 1990 and has been working since at the Laboratoire de Chimie de la Matière Condensée de Paris (LCMCP-UMR 7574). In 2010, F. Ribot obtained his habilitation to lead research from Pierre et Marie Curie University (UPMC). He co-authored more than 100 peer reviewed papers.
His research deals with the chemistry of materials. He has contributed to the fields of sol-gel by studying the role of complexing ligands on the reactivity of various metals alkoxydes. He then worked on hybrid organic-inorganic materials and more particularly on those designed by the nanobulding blocks approach from organotin oxo-clusters. He also collaborated with industrial partners on the nanotexturation of hybrid coatings and the controlled precipitation of cerium(IV) phosphates. Over the last years, he shifted his research focus toward gold nanoparticles with two main topics: the synthesis of N-heterocyclic carbene stabilized gold nanoparticles and the in-situ study of their functionalization.
NMR spectroscopy constitutes an important tool in his researches and he developed strategies based on diffusion ordered spectroscopy (DOSY) experiments to study the interface of various nano-objects, ranging from molecularly defined oxo-clusters to nanoparticles, in solution or in suspension.
Last update 2020/01/07
- Alexandre Porcheron, « Coordination complexes grafted on plasmonic nanoparticles », Sorbonne Université, started October 2018 – financed by ANR COCOsMEN, PhD adviser, co-supervised with L. Fensterbank.
- Victoire Asila, « N-heterocyclic carbene-stabilized gold nanoclusters », Sorbonne Université, started October 2018 – financed by ED397, co-supervised with C. Chanéac (PhD adviser).
- Laura Hippolyte, « New syntheses of N-heterocyclic carbene-stabilized gold nanoparticles », UPMC, 26/11/2018 – financed by Labex MiChem, ED 397 and Labex Matisse, PhD adviser, co-supervised with L. Fensterbank.
- Perrot Alexandre, « Sol-gel coatings incorporating active mesostructured particles obtained by spray drying for aeronautical aluminum alloys protection against corrosion », UPMC, 2/6/2015 – financed by ANR AERO2, PhD adviser, co-supervised with L. Nicole.
- Ben Sassi Hanen, « Probing, in suspension, the ligands on the surface of gold nanoparticles by DOSY NMR », UPMC, 28/2/2013 – financed by MESR, PhD adviser.
- Letailleur Alban, « Pattering of hybrid silica coatings by nanoimprint : applications to light extractions », UPMC, 15/3/2012 – financed by Saint-Gobain Recherche, co-supervised with C. Boissière and C. Sanchez (PhD adviser).
- Monget Julie, « Hybrid sol-gel coating for the corrosion protection of aeronautical aluminium alloys », UPMC, 14/12/2010 – financed by EADS-IW, co-supervised with L. Nicole and C. Sanchez (PhD adviser).
- Nazaraly Micaela, « Control of structure, morphology and size of cerium(IV) phosphate particles », UPMC, 24/10/2006 – financed by Rhodia, co-supervised with C. Chanéac and J.-P. Jolivet (PhD adviser).
- De Monredon Sophie, « Organosilanes/precipited silica interaction from hydroalcoolic to aqueous medium », UPMC, 7/12/2004 – financed by Rhodia, co-supervised with C. Bonhomme and F. Babonneau (PhD adviser).
- Eychenne-Baron Christophe, « New syntheses of the macrocation {(BuSn)12O14(OH)6}2+: use as nanobrick for the synthesis. of hybrid materials », UPMC, 11/5/2001 – financed by MRT, co-supervised with C. Sanchez (PhD adviser).
- Banse Frédéric, « Hydrolysis-condensation of monorganotin trialkoxides: synthesis of hybrid organic-inorganic systems », UPMC, 20/10/1995 – financed by MRT, co-supervised with C. Sanchez (PhD adviser).
Last update 2020/01/05
- Nathalie Bridonneau, « Synthesis of N-heterocyclic carbene-stabilized gold nanoparticles », 1/9/2016-31/8/2017, ATER UPMC.
- Anne Soleilhavoup, « Probing, by multinuclear NMR, the integrity and mobility of organotin oxo-clusters inserted in lipidic membranes », 5/1/2015-29/2/2016, ANR MEMINT.
- Baradari Hiva, « Mineral and hybrid additives for Portland cement », 15/2/2012-31/8/2013, financed by Chryso.
- Becuwe Matthieu, « Portland cement based hybrid materials », 1/9/2010-31/8/2011, financed by Chryso.
- Van Lokeren Luk, « Pulsed field gradient based NMR methodology to probe, in suspension, the affinity of an organic ligand for nanoparticles surface », 1/9/2009-29/2/2012, ANR EVALON (ANR-08-NANO-026).
- Van der Beek David, « New hybrid organic-inorganic liquid crystals based on nano building units », 2/11/2005-15/7/2007, EU(FP6-Marie Curie Mobility Actions) HYBRIDLICRYST.
- Martinez-Ferrero Eugenia, « New tin(IV) based-nanobricks for hybrid materials », 1/10/2003-31/1/2005, EU(FP5-HP) NBB-Hybrids.
- Armelao Lidia, « Sol-process prepared tin oxide », 1/4/1995-31/3/1996, grant from the University of Padova (Italy).
- Kehr Gerald, « Monoorganotin based hybrid systems », 3/4/1995-2/2/1996, EU(FP3-HCM) CHRX940610.
Last update 2020/01/05
- L. Angiolini, D. Caretti (Universita degli Studi di Bologna, Bologne, Italy): Organotin based hybrid materials
- D. Constantin, B. Pansu, (LPS – UMR 8502, Orsay): Inclusions in lipid membranes, gold nanoparticles
- D. Dakternieks (Deakin University, Geelong, Australia): Organotin oxo-clusters chemistry
- S. Diré (Universita di Trento, Trente, Italy): Silsesquioxane based NBB and hybrid materials
- B. Dubertret, N. Lequeux, T. Pons (LPEM – UMR 8213, ESPCI, Paris): Probing the functionalization of QD by NMR
- L. Fensterbank, B. Fleury, D. Lesage (IPCM – UMR 8232, Sorbonne-Université, Paris): NHC stabilized gold nanoparticles and clusters
- B. Jousseaume, T. Toupance (ISM – UMR 5255, Université de Bordeaux, Talence): Organotin based hybrid materials
- M. Kahn, B. Chaudret (LCC – UPR 8241, Toulouse): Probing the functionalization of nanoparticles by DOSY NMR
- T. Lalot (HiCSA – EA 4100, Panthéon Sorbonne, Paris): Hybrid materials
- M. Lejeune (IRCER – UMR 7315, Université de Limoges, Limoges): 29Si NMR characterization of sol-gel inks
- J.C. Martins (Ghent Universiteit, Ghent, Belgium): Advanced 119Sn NMR and DOSY NMR
- L. Matejka, A. Strachota (IMC, Prague, Czech Republic): Organotin oxo-clusters based hybrid materials
- D. Mercier (IRCP – UMR 8247, ENSCP, Paris): NHC modified surfaces
- N. Mézailles (LHFA – UMR 5069, Université Paul Sabatier, Toulouse): DOSY NMR
- G. Morris (Manchester University, Manchester, UK): DOSY NMR
- A.-L. Rollet (PHENIX – UMR 8234, Sorbonne Université, Paris) : DOSY NMR with uncommon nuclei
- C. Schatz (LPCO – UMR 5629, ENSCBP, Bordeaux): DOSY NMR on polyelectrolytes solutions and coacervates
- N. Steunou (ILV – UMR 8180, Université Versailles Saint Quentin en Yvelines, Versailles): Vanadium oxide nanotubes
- O. Tillement (ILM – UMR 5306, Université Claude Bernard, Villeurbanne): ultra-small multifunctional Gd-based silica nanoparticles
- R. Willem (Vrije Universiteit Brussel, Brussels, Belgium): Advanced multinuclear NMR
Last update 2020/01/07
The macrocation {(RSn)12O14(OH)6}2+
Various organizations of {(BuSn)12O14(OH)6}2+ encountered in the solid state.

In {(BuSn)12O14(OH)6}(pTS)2(C4H8O2)2, the chains, formed by bridging p-toluenesulfonate anions, are assembled in plane by dioxane molecules, which interact with five-coordinated tin atoms (Organometallics 2000, 19, 1940-1949).
In {(BuSn)12O14(OH)6}(CHAPS)2, the chains, formed by a complex set of electrostatic contacts between the 3-cyclohexylamino-1-propanesulfonate anions and the µ2-OH groups of the macrocation, are assembled in planes through hydrogen bonds involving the amino and the sulfonate groups (unpublished result).
In {(BuSn)12O14(OH)6}(AMPS)2, -NH•••O=C(R)-NH•••O=C(R)- hydrogen bonds yield right and left helices, which are assembled in a 3D framework by a complex set of electrostatic contacts between the 2-acrylamido-2-methyl-1-propanesulfonate anions and the µ2-OH of the macrocation (J. Mater. Chem. 2005, 15, 3973-3978).
Sn12(μ3-O)6(μ2-O)2(μ2-OH)4(μ2-OEt)10(OEt)18(HOEt)4

Last update 2020/01/05
Strategies to functionalise and assemble the macrocation {(RSn)12O14(OH)6}2+

Last update 2020/01/05
Coordination complexes grafted on metallic nanoparticles: toward synergy
N-heterocyclic carbene-stabilized gold nanoparticles

Quantified binding scale of competing ligands on the surface of thiols stabilized gold nanoparticles
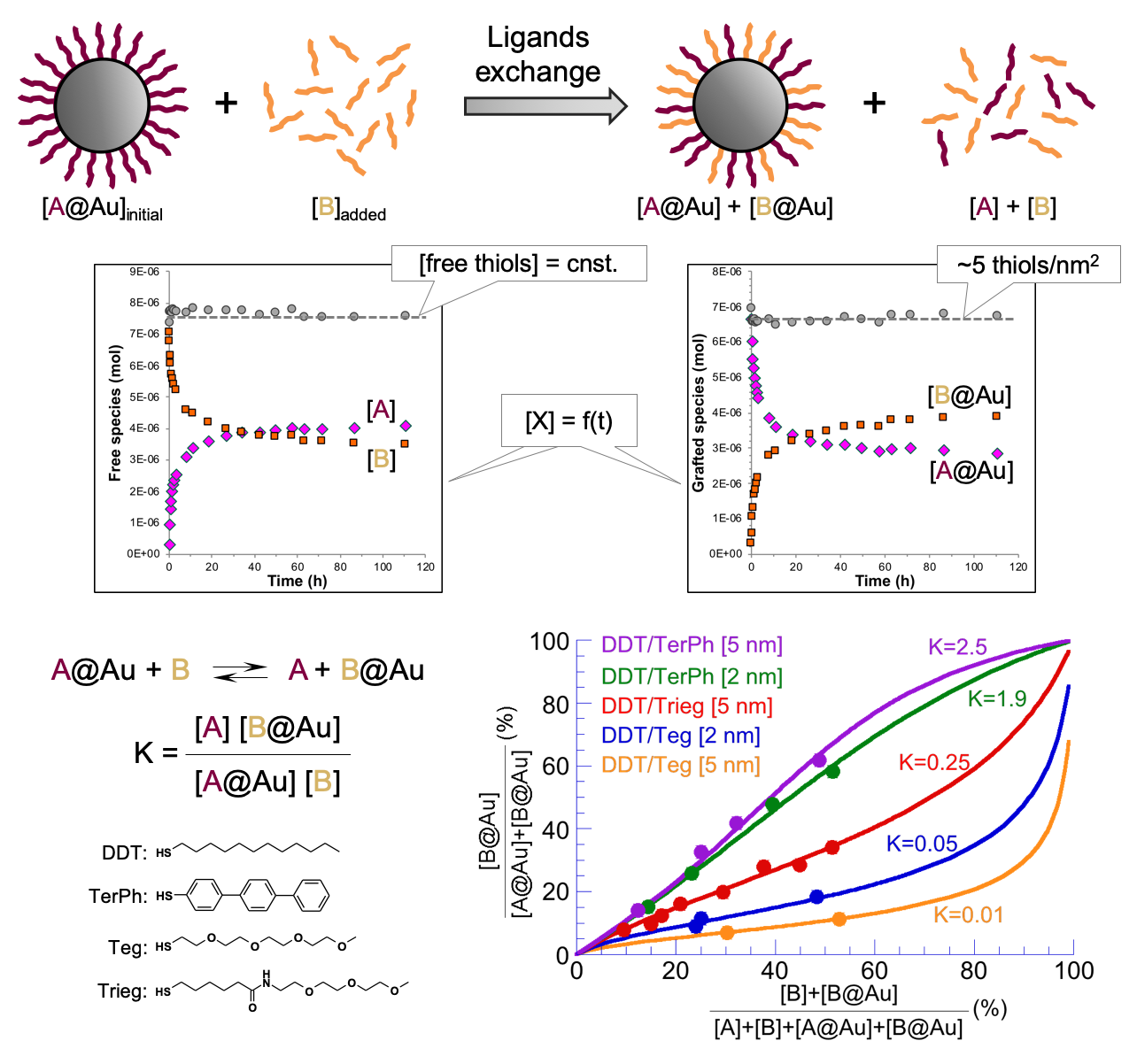
Last update 2020/01/05
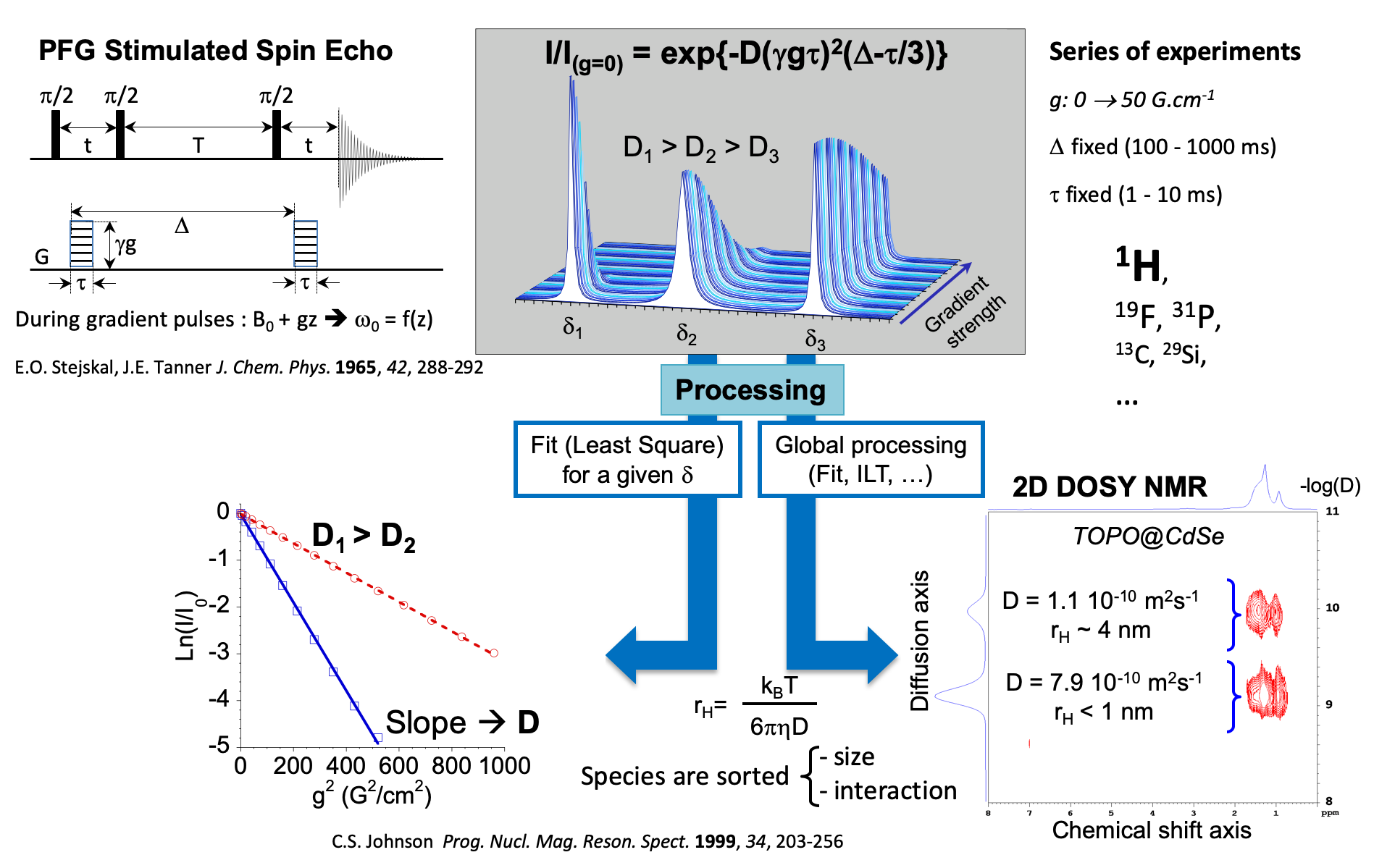
Pulsed Field Gradient NMR basis
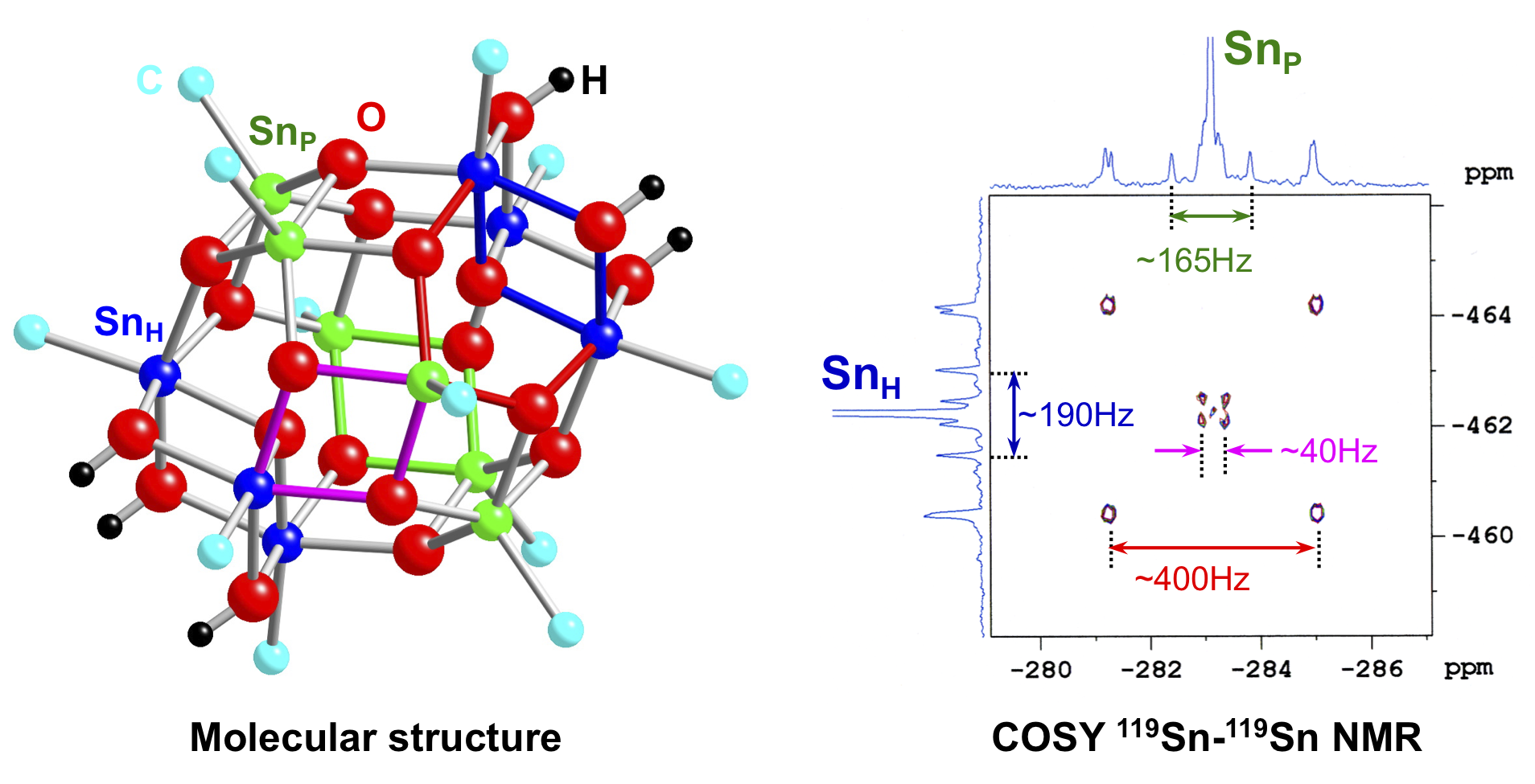
Probing the ionic association on {(BuSn)12O14(OH)6}X2

Looking at the functionalization of Ti16O16(OEt)32
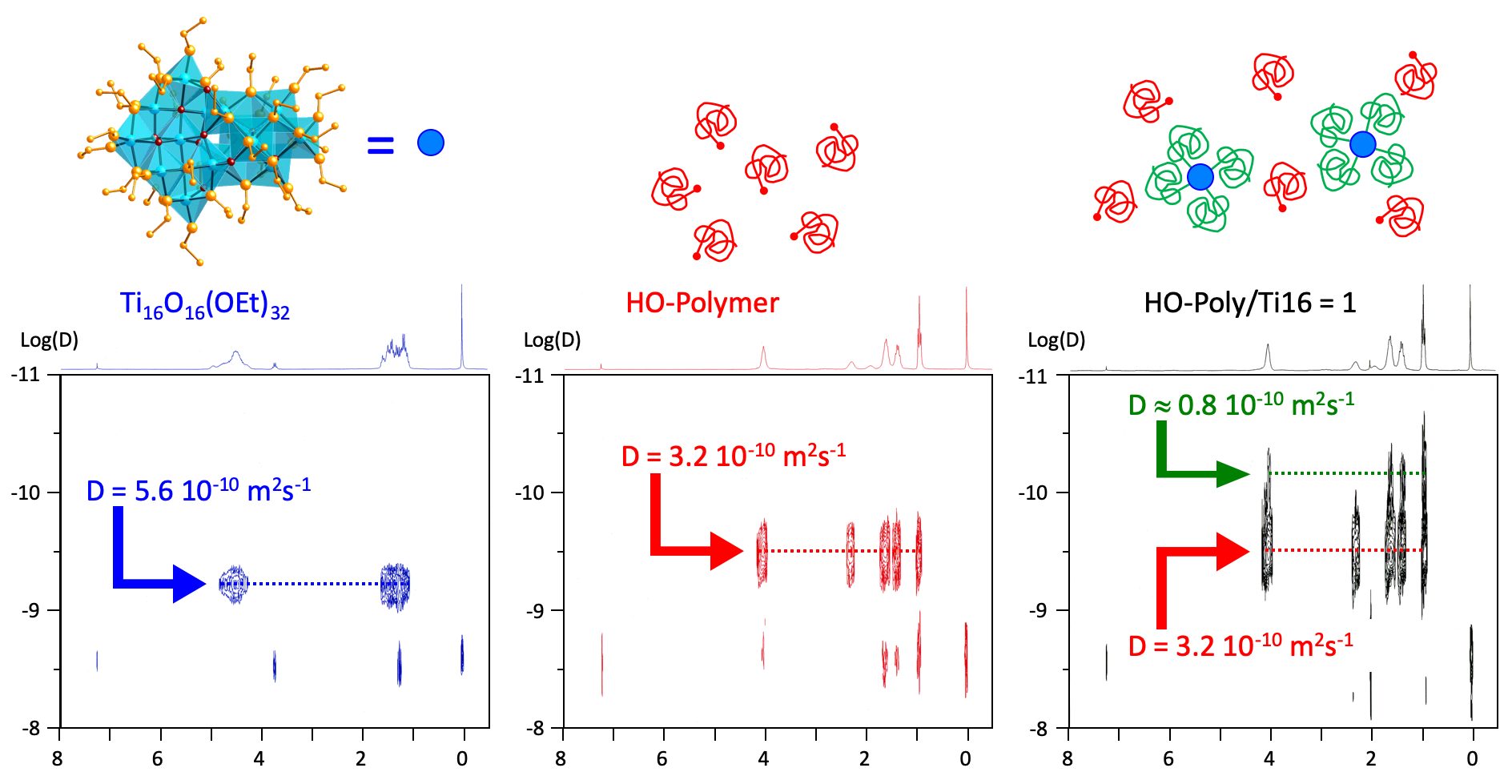
Ligands interacting with sol-gel prepared nano-TiO2
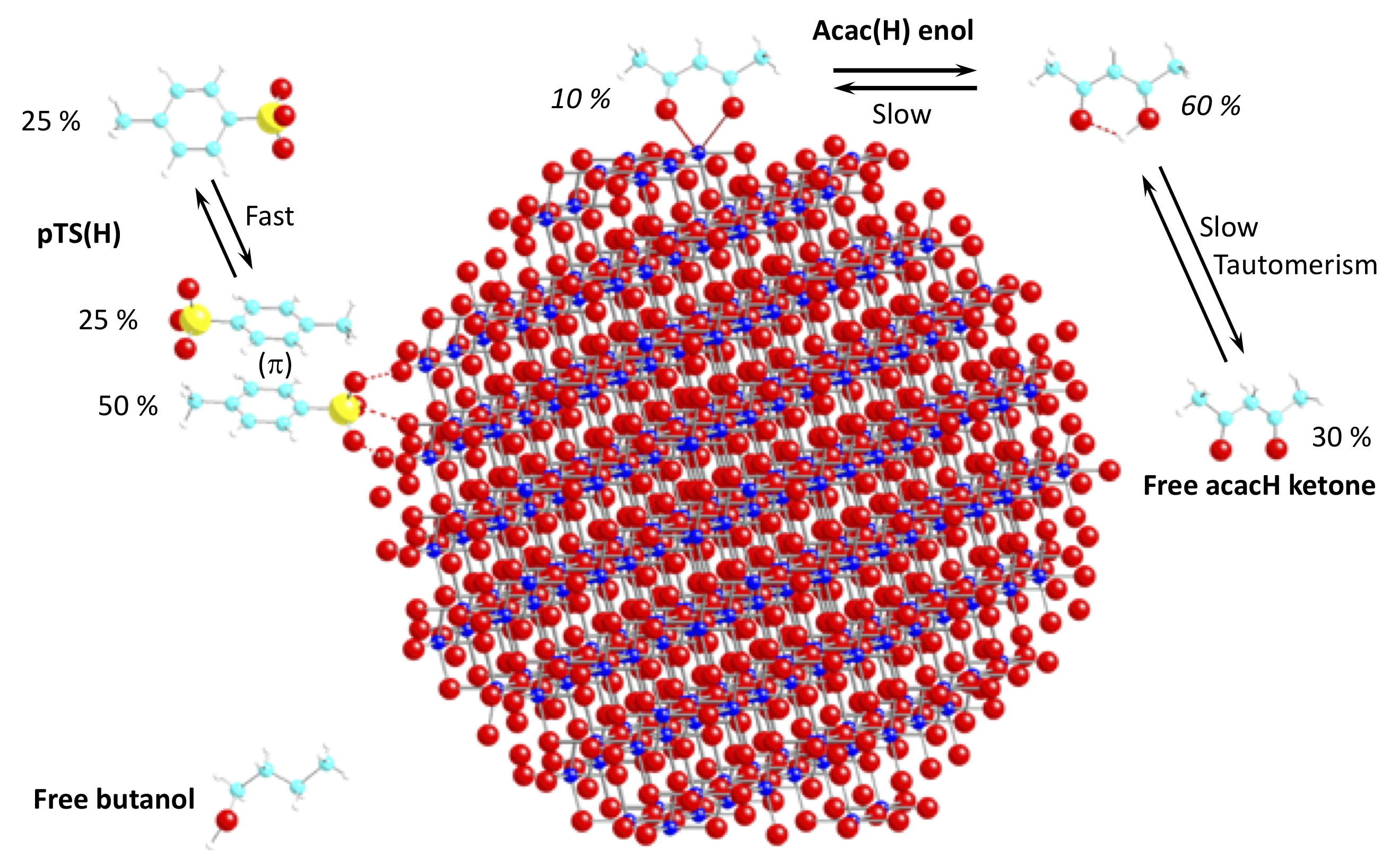
Quantitative analysis of mixtures
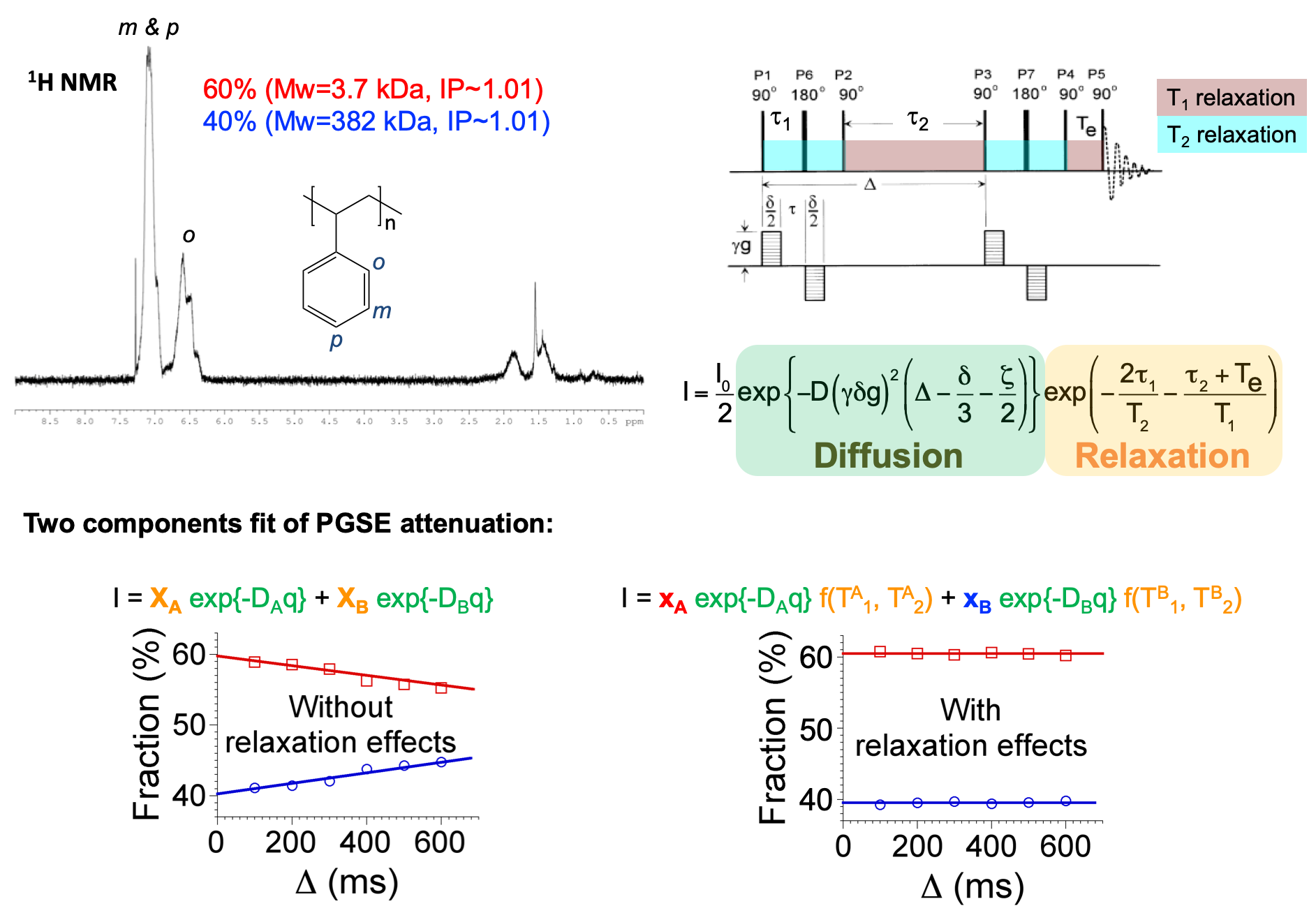
Pulsed Field-Gradient Spin Echo (PGSE) NMR, which associates to a spectral dimension the measure of diffusion coefficients, is a convenient technique for mixture analysis. Unfortunately, because of relaxation effects, the quantification by PGSE NMR is far from straightforward for mixtures with strong spectral overlap. By explicitly including the relaxation effects in the Stejskal and Tanner equation, meaningful fractions can be obtained from a multicomponent fit of the PGSE attenuation. This approach is illustrated with a binary mixture of polystyrenes, the 1H NMR spectra of which are perfectly identical. Fractions with error lower than 3% are obtained. The T1 and T2 values, required for this analysis, are either independently measured with conventional sequences or determined, along with the fractions and the diffusion coefficients, from the simultaneous analysis of up to 6 PGSE data sets recorded with different diffusion delays (J. Magn. Reson. 2013, 231, 46-53).
Dynamics at the cross-linking nodes in hybrid self-healing materials

Last update 2020/01/06
None for the moment
Last update 2020/08/03
Last update on 2020/08/03















